World Health Organization (WHO) officials reeling under the onslaught of COVID-19 are generally encouraged by recent vaccine developments from companies such as Pfizer, BioNTech and Moderna.
Yet they come with the caveat that there is still a long way to go in the struggle against the novel coronavirus.
Patience and vigilance against the virus are needed going forward, and factors around a vaccine such as cost, distribution and storage temperature will need to have answers.
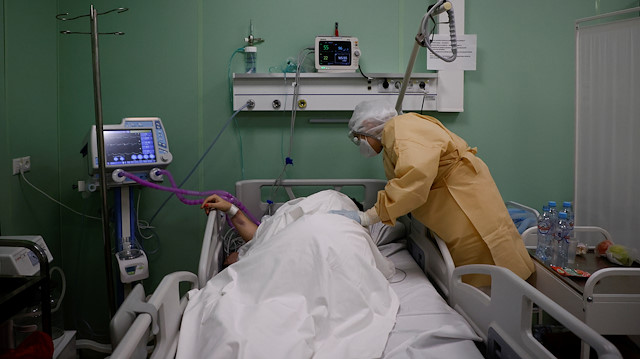
COVID-19 has infected more than 56 million people worldwide and claimed the lives of over 1.34 million people.
In all countries, people have to take a host of other measures to prevent the virus's spread before vaccines move into the mainstream, say WHO scientists.
US drugmaker Pfizer and German biotech company BioNTech said Wednesday that their coronavirus vaccine is 95% effective, upgrading an earlier estimate of 90%.
They said their clinical trial had now been completed and they would apply soon for regulators' approval to give the vaccine to the public.
Their announcement comes days after US drug company Moderna said its own shot was 94.5% effective.
"It is encouraging to hear the announcement from Moderna on the efficacy results of their vaccine candidate and the information about the stability at standard refrigeration temperatures," WHO spokesman Tarik Jašarević told Anadolu Agency.
"We are also encouraged by the robust vaccine development pipeline globally, with 47 candidates in clinical development, including 10 in phase III clinical trials."
Jašarević said the WHO is looking forward to the results "of rigorous, well-designed, well-conducted clinical trials" that will show the candidates' worthwhile efficacy and the power to prevent the COVID-19 disease, with safety and duration of protection.
He said there are many challenges to overcome, including to produce enough doses and ensure fair and equitable access for people everywhere.
"This will take time. Therefore, we must continue to use public health tools and measures that we know are effective in preventing the infection and breaking the chains of transmission," noted Jašarević.
He echoed words earlier this week from WHO Director-General Tedros Ghebreyesus, who also said now is no time for complacency.
"While we continue to receive encouraging news about COVID-19 vaccines and remain cautiously optimistic about the potential for new tools to start to arrive in the coming months, right now, we’re extremely concerned by the surging cases we are seeing in some countries,” said Tedros, particularly in Europe and the Americas.
Tedros also said that health workers and health systems are being pushed to the breaking point.
The WHO has issued guidance and tools to increase medical and public health workforce supplies and facilities to manage COVID-19 patients.
The WHO's chief scientist, Dr. Soumya Swaminathan, told journalists this week that it was "very encouraging" to see that the Pfizer and Moderna vaccines "seem to be achieving high efficacy."
"It's quite encouraging. We have just heard about the interim results from the press release from Moderna.
"Of course, we need to wait and see what the final efficacy and the safety profile of this vaccine will be when the whole data is analyzed after they reach their primary endpoint," said Swaminathan.
There would have to be "enough follow-up" of at least two months for the trial participants, for the side effects, and then the drug can be submitted to the regulatory agencies.
The WHO chief scientist said the COVAX facility is to facilitate this discussion with vaccine manufacturers worldwide.
"We are open to the procurement of vaccine doses, that are criteria of course for the procurement, and there is an independent privatization group that's been set up that will look at the dossiers, that will look at the data that manufacturers are submitting," said Swaminathan.
"And then there are the cost considerations as well, the affordability, and then there are practical considerations, like the need for cold storage."
She said the number of doses of vaccine that will be required could be available early in 2021.
Swaminathan said that "one very encouraging thing is to see that at least two vaccine makers "seem to be achieving high efficacy."
"But there are many, many questions still remaining about the duration of protection," she said, as well as the “impact on severe disease, the impact on different subpopulations, especially the elderly, and the adverse events, beyond a certain period of time."
Swaminathan said the clinical trials will continue to collect data because "that's really important for us to know about the long term."
Dr. Kate O'Brien, the WHO's director of the Department of Immunization, Vaccines and Biologicals, said information from Moderna that its vaccine might need refrigeration at only minus 20 C is "welcome news."
"And we will be looking really carefully at the ease with which different vaccines can be delivered and certainly about the number of doses that are required," said O'Brien.
"And certainly any vaccine that can achieve a one-dose vaccine is certainly easier to deliver than a two-dose vaccine," she explained.
"And of course, the cold chain characteristics are also important. So information from the Modena vaccine about its requirements of both minus 20 and then refrigeration, for a period…is welcome news as well."
Pfizer had said its vaccine would have to be stored at minus 75 C, making it very difficult to stock.